R&D Grade mRNA Manufacturing
mRNA manufacturing workflow by in vitro transcription
mRNA is attracting attention as an innovative treatment for a wide range of diseases. Our mRNA synthesis service is optimized from template plasmid design and construction to mRNA synthesis by IVT using our unique platform.
-

mRNA
design support -

Gene synthesis
-

Optimization
-

Manufacturing
-

LNP
Customized mRNA synthesis to meet your needs
Our services can be flexibly customized to meet the requirements of your project. By designing and optimizing mRNA in the early stages of drug discovery, we ensure a seamless transition to scale-up and GMP manufacturing.

| 5’Cap | ORF | Modified nucleic acid | UTR | Poly A |
|---|---|---|---|---|
| ・CleanCap ® M6 ・CleanCap ® AG ・CleanCap ® AG 3’OMe ・Enzymatic capping ・Uncapped | ・Catalog sequence ・Customer’s sequence ・Codon optimization ・Linker strategies | ・N1-Methyl-Pseudo-UTP ・5-Methoxy-UTP ・Pseudo-UTP ・5-Methyl-UTP ・5-Methyl-CTP ・Unmodified | ・Elixirgen UTRs ・Customer’s UTRs ・UTR optimization | ・120 nt A tail ・100 nt A tail ・80 nt A tail ・Custom tail length ・Linker strategies |
*Our company has licensed the innovative 5' cap addition technology “CleanCap®” from TriLink Biotechnologies, Inc. (hereinafter referred to as “Trilink”).
Other options
| Purification | LNP |
|---|---|
| ・Silica column + centrifugal purification ・Oligo dT method ・Reverse phase LC method ・Other (please consult us) | ・Standard recommended prescription ・Customer prescription |
Various optimization options

Capping
By adding a cap structure to the 5′ end of mRNA molecules, resistance to degradation by exonucleases is greatly improved, resulting in significantly enhanced stability and translation efficiency within cells. The enzymatic method offers high flexibility, and CleanCap® achieves high purity and high capping efficiency in a single step.
Modified nucleic acid
By chemically modifying bases such as uridine, immune induction by pattern recognition receptors (TLRs, etc.) is suppressed, minimizing inflammatory responses. In particular, UTP modification reduces immunogenicity without compromising translation efficiency and improves the sustainability of protein expression.
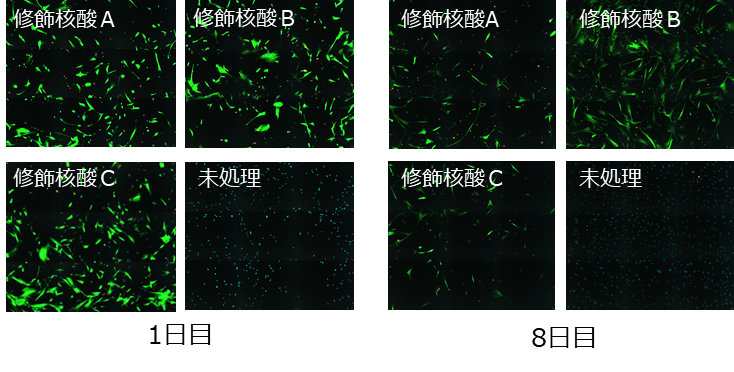
In human fibroblasts, strong GFP fluorescence was observed even after 8 days using synthetic GFP mRNA containing modified nucleic acid A.

UTR optimization
By optimizing the sequence and secondary structure of the 5'- and 3'-untranslated regions (UTRs), ribosome binding and recycling efficiency are improved, thereby extending mRNA stability and translation duration. Proper placement of stabilization signals and RNA-binding protein recognition sites is key.
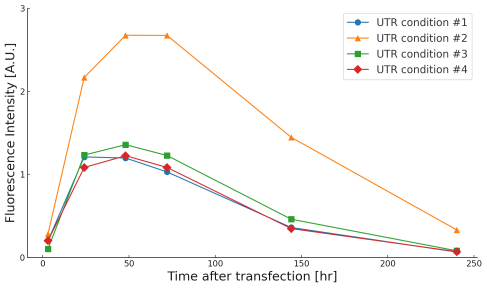
Cell: HEK293
CDS: eGFP
Cap: CleanCap® Reagent AG (3’OMe)
Modified nucleic acid: N1-Methyl-ΨU

Codon optimization
By replacing codons in the amino acid sequence of the target protein with those that are frequently used in the host cell, the translation speed of the ribosome is increased, maximizing protein production. GC content and secondary structure are also taken into consideration to achieve both translation efficiency and mRNA stability.
Purification
By thoroughly removing impurities such as incomplete transcription products, dimers, and DNA template residues, we reduce the immune response and cytotoxicity induced, ensuring safety and reproducibility. We select purification processes appropriate for the application, such as the oligo-dT method or reverse-phase LC method.

LNP formulation
Encapsulating mRNA in lipid nanoparticles (LNP) protects it from degradation by enzymes in the bloodstream and intercellular spaces, significantly improving uptake efficiency into target cells. LNP promotes endosomal escape after internalization and optimizes mRNA release into the cytoplasm.
Service Package
We smoothly provide the necessary format and quantity of mRNA according to your application needs.

Discovery Package: Small-lot, high-mix production for initial screening purposes
| Volume | 100 μg (Guaranteed yield: 50 μg *1) |
|---|---|
| Capping | CleanCap AG-3'OMe recommended *1 |
| Modified Nucleic Acid | N1-Methyl-Pseudo-UTP recommended *1 |
| Template | Customer-supplied or package including template synthesis costs |
| QC | Concentration test (NanoDrop) / Integrity test (CE) |
| Price | IVT only 100,000 JPY / Template synthesis + IVT 200,000 JPY |
Research Package: Non-GMP Manufacturing for Vitro/Vivo Experiments
| Volume | 0.5–100 mg (Guaranteed yield: 50% of target) |
|---|---|
| Capping | CleanCap or Enzymatic Capping *1 |
| Modified Nucleic Acid | Various UTP modifications recommended *1 |
| Template | Package including template synthesis costs |
| QC | Concentration test (NanoDrop)/ Integrity test (CE)/ Endotoxin, etc. |
| Price | Inquiry |

Preclinical Package: Non-GMP manufacturing for toxicological purposes
| Volume | 100–1,000 mg (Guaranteed yield: 80% of target) |
|---|---|
| Capping | CleanCap or Enzymatic Capping *1 |
| Modified Nucleic Acid | Various UTP modifications recommended (consultation available) |
| Template | Template synthesis costs included in package |
| QC | Test items comply with USP 3.0 *1, 2 |
| Price | Inquiry |
*1 Consultation available
*2 As of May 2025
Quality Contorol
We offer mRNA quality testing. We utilize domestic facilities for mRNA drug-specific analysis items, enabling quick and flexible response.
(Available as an additional option for mRNA synthesis.)
| Quality | Attribute | Method | Discovery Pack | Research | Pre-clinical | |
|---|---|---|---|---|---|---|
| Identity | Sequence verification | Sanger sequence | - | - | OK | |
| Content | RNA content measurement (concentration, A260/A280 ratio) | Absorbance (UV) | OK | OK | OK | |
| Integrity | mRNA integrity | Capillary gel electrophoresis (CGE) | OK | OK | OK | |
| Purity | Purity verification | To be discussed | - | - | - | |
| 5' capping efficiency | LC-MS | - | OK | OK | ||
| 3' poly(A) tail length | LC-MS | - | - | OK | ||
| Impurities originating from the product | dsRNA | ELISA | - | OK | OK | |
| Aggregation rate | To be discussed | - | - | - | ||
| Short-chain contamination rate | To be discussed | - | - | - | ||
| Residual DNA template | qPCR | - | - | OK | ||
| Residual nucleotides | To be discussed | - | - | - | ||
| Residual reagents, etc. | To be discussed | - | - | - | ||
| Residual T7 polymerase | ELISA | - | - | OK | ||
| Potency | Protein expression | To be discussed | - | - | - | |
| Safety | Endotoxin test | USP <85>、JP<4.01> | - | OK | OK | |
| Microbial limit test | USP <61>, <62>, <1115> JP<4.05> | - | - | OK | ||
| Other | Properties confirmation | USP <790> | OK | OK | OK | |
| Residual solvent | USP <467>、JP<2.46> | - | - | OK | ||
| pH | USP <791>、JP<2.54> | - | - | OK | ||
Ref. : Analytical Procedures for Quality of mRNA Vaccines and Therapeutics (Draft Guidelines: 3rd Edition)
*Please contact us if you would like additional quality control and evaluation.